

In the medical field, wound healing remains a critical challenge. The process is inherently slow and complex, requiring meticulous care to prevent infection, alleviate pain, and minimize scarring. Dressings play a pivotal role by protecting wounds, absorbing exudate, and maintaining an optimal moist environment. Despite existing materials, the quest for safer and more effective alternatives persists.
AMS BioteQ’s SIPSIP Pro Gelatin Dressing exemplifies such innovation. Gelatin, a natural and biodegradable material with exceptional biocompatibility, has gained attention for its porous structure that absorbs exudate and supports tissue regeneration. However, its susceptibility to degradation in moist environments limits durability. To address this, AMS BioteQ collaborated with National Taiwan University of Science and Technology and Tokushima University (Japan), developing a gelatin dressing crosslinked with glutaraldehyde and processed via freeze-drying. The resulting foam features uniformly distributed pores (60–70 μm average size), enabling superior fluid absorption.
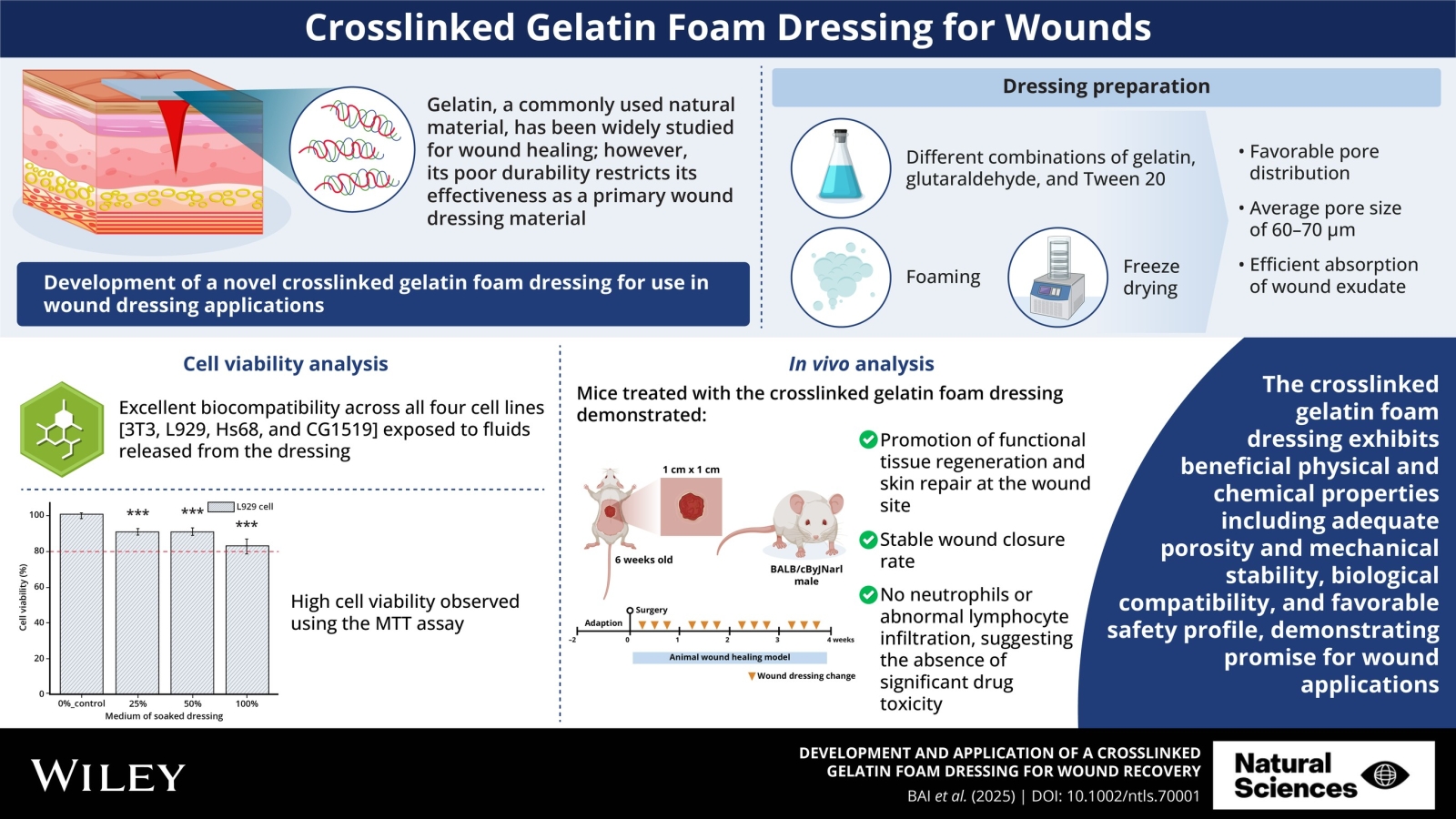
- Safety & Efficacy:
- In vitro tests on four cell lines (3T3, L929, Hs68, CG1519) demonstrated >80% cell viability, confirming low toxicity and high biocompatibility.
- In vivo studies on murine full-thickness wounds showed accelerated tissue regeneration compared to leading commercial dressings, with no signs of hepatic or renal toxicity.
These findings position gelatin dressings as a safe, eco-friendly alternative for wound care, with potential to redefine standards through further clinical validation.
Market Potential
The global medical dressing market is expanding rapidly:
- Valuation:
- 2023: $8.6 billion (MarketsandMarkets, 2023)
- 2028 Projection: $11.5 billion (6.0% CAGR), driven by aging populations and rising chronic wounds.
- Asia-Pacific Growth: >6.5% annual growth (Allied Market Research, 2023), fueled by diabetes prevalence and healthcare advancements.
- Innovation Demand: Frost & Sullivan (2022) estimates >25% market share for advanced dressings (e.g., biomaterials, smart dressings) by 2028.
SIPSIP Pro aligns with these trends, offering:
✅ High biocompatibility
✅ Sustainable material sourcing
✅ Competitive performance metrics
AMS BioteQ (6864-TW) plans continued R&D investment to broaden clinical applications and market penetration.
Future Outlook
Chairman Tsai Yi-Ju emphasizes AMS BioteQ’s commitment to advancing wound care technology. SIPSIP Pro represents both a corporate milestone and a medical breakthrough, with potential to enhance patient outcomes globally.
Resources
- Product Video: SIPSIP Pro Introduction
- Website: AMS BioteQ Official Site
- Research: Published in Natural Science; full report available upon request.
References
- MarketsandMarkets. (2023). Medical Adhesive Tapes and Bandages Market Report.
- Allied Market Research. (2023). Asia-Pacific Advanced Wound Care Analysis.
- Frost & Sullivan. (2022). Global Wound Care Solutions Outlook.