
(1).jpg)
To meet the demand for high-quality wound care products in the Japanese market, AMS BioteQ (Stock Code: 6864-TW) has continued to deepen its overseas medical device strategy. On the 23rd of this month, the company announced that two of its foam wound dressing products have officially passed the review by Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), obtaining the sales license for Class I Medical Devices (General Medical Devices). This certification will accelerate AMS BioteQ’s progress in the Japanese market, strengthen its regulatory and technical capabilities in the Asian medical device market, and inject momentum into future revenue growth and international licensing strategies.
AMS%20BioteQ%E4%BC%81%E6%A5%AD%E4%BB%8B%E7%B4%B9%EF%BC%882025Q3.pptx.jpg)
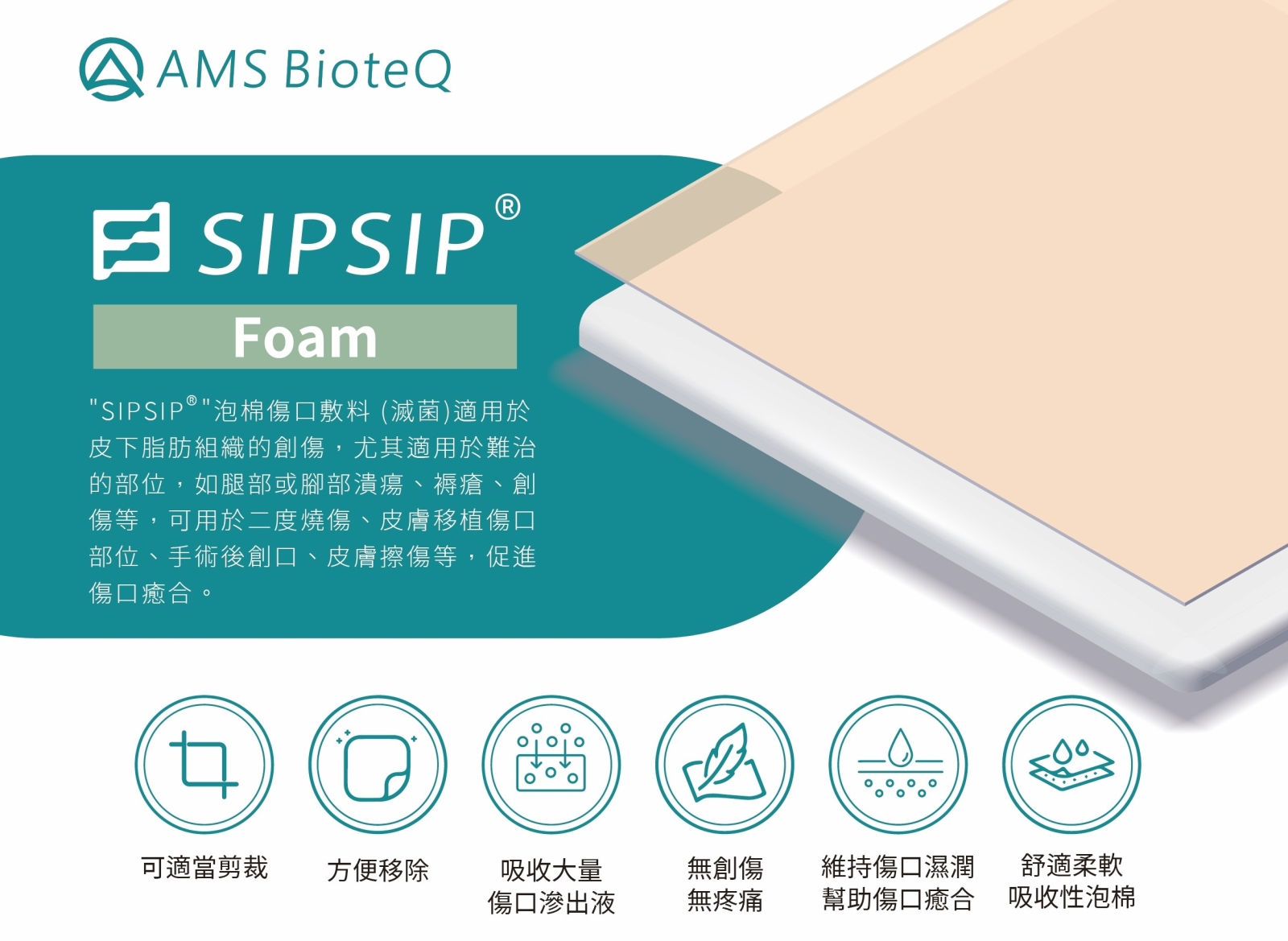
The SIPSIP series of foam wound dressings are designed to provide a moist healing environment. The Foam variant features a self-adhesive design with high absorbency and anti-adhesion properties, effectively absorbing wound exudate, reducing discomfort during dressing changes, and preventing secondary trauma. It also maintains wound moisture and cleanliness, promoting faster healing and enhancing patient comfort. The FoamLite variant, designed without a waterproof membrane, can be used in conjunction with Negative Pressure Wound Therapy (NPWT) devices to improve the efficiency of care for deep or chronic wounds. Both products are suitable for use in medical institutions and home care settings, including for leg ulcers, pressure sores, surgical wounds, second-degree burns, and graft sites. They hold significant potential for clinical and long-term care institutions.
According to a 2024 report by market research firm IMARC Group, the Japanese wound care market has reached a size of 746millionUSD746millionUSD, with an estimated growth to 1.127billionUSD1.127billionUSD by 2033, representing a compound annual growth rate (CAGR) of 4.3% from 2025 to 2033. Among wound care categories, foam dressings (Foam Dressing) are considered one of the most promising, primarily due to their ability to enhance dressing change comfort, reduce infection risks, and their high compatibility with elderly and chronic care environments. Market Research Future (MRFR) reports that the Asia-Pacific foam dressing market will maintain an annual growth rate of over 5% by 2030, with Japan, due to its aging population and increasing medical expenditures, remaining one of the region’s most important growth drivers. AMS BioteQ’s PMDA certification effectively opens the door to mainstream medical device distribution in the region, significantly boosting future revenue and international licensing prospects.
AMS BioteQ Chairman Tsai Yi-Ju stated that Japan is a highly aging society with significant demand for medical devices, particularly chronic wound care products. The SIPSIP series of foam dressings can effectively improve the quality of dressing changes, reduce the burden on caregivers, and provide a safer and more effective option for burn, pressure sore, and post-surgical wound healing. This product line fills a specific gap in the Japanese market for advanced wound dressings. He further noted that while the Japanese market has stringent regulations and high entry barriers, it is highly open to innovative technologies. Passing PMDA certification not only signifies product maturity but also represents international recognition of quality, setting a benchmark for future expansion into European, American, and Asia-Pacific markets.
AMS BioteQ emphasized that the successful registration of these two dressings not only signifies compliance with Japan’s strict medical device standards but also paves the way for the extension and licensing of future product lines. The company will continue to develop advanced foam dressing products with antibacterial, hemostatic, and multi-functional properties in response to market characteristics and clinical needs, while simultaneously applying for overseas medical device certifications. AMS BioteQ is actively advancing global licensing and product commercialization processes. In collaboration with local partners and distribution resources in Japan, the company also plans to introduce a more comprehensive product line portfolio to expand business collaborations and brand presence. With this successful PMDA registration and sales license, AMS BioteQ has successfully entered Japan’s high-growth potential elderly medical device market, demonstrating Taiwan’s competitive strength and long-term vision in the global medical device market.